
Complete 360° range of motion
The articulated arm, which is included in the system scope, enables the sensor of the stepper to be positioned anywhere within the room. Its six joints allows for 360° movement in any direction.

Fine positioning
Lifting and rotating on the handle of our MTT SolidLock HS articulated arm allows you to further perform light adjustments. This allows you to effortlessly raise and shift the ultrasonic probe.
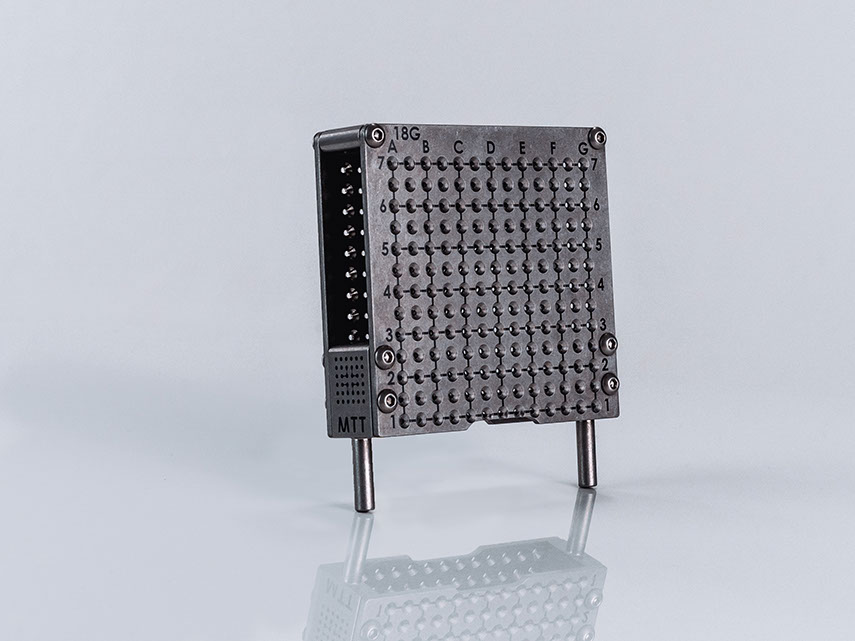
Accurate placement using templates
Our SoLo A is engineered for the use of guide grids, such as our PeriGrid, to guarantee the secure and precise positioning of needles. The supplied template holder is attached to the front of the stepper motor for this purpose.
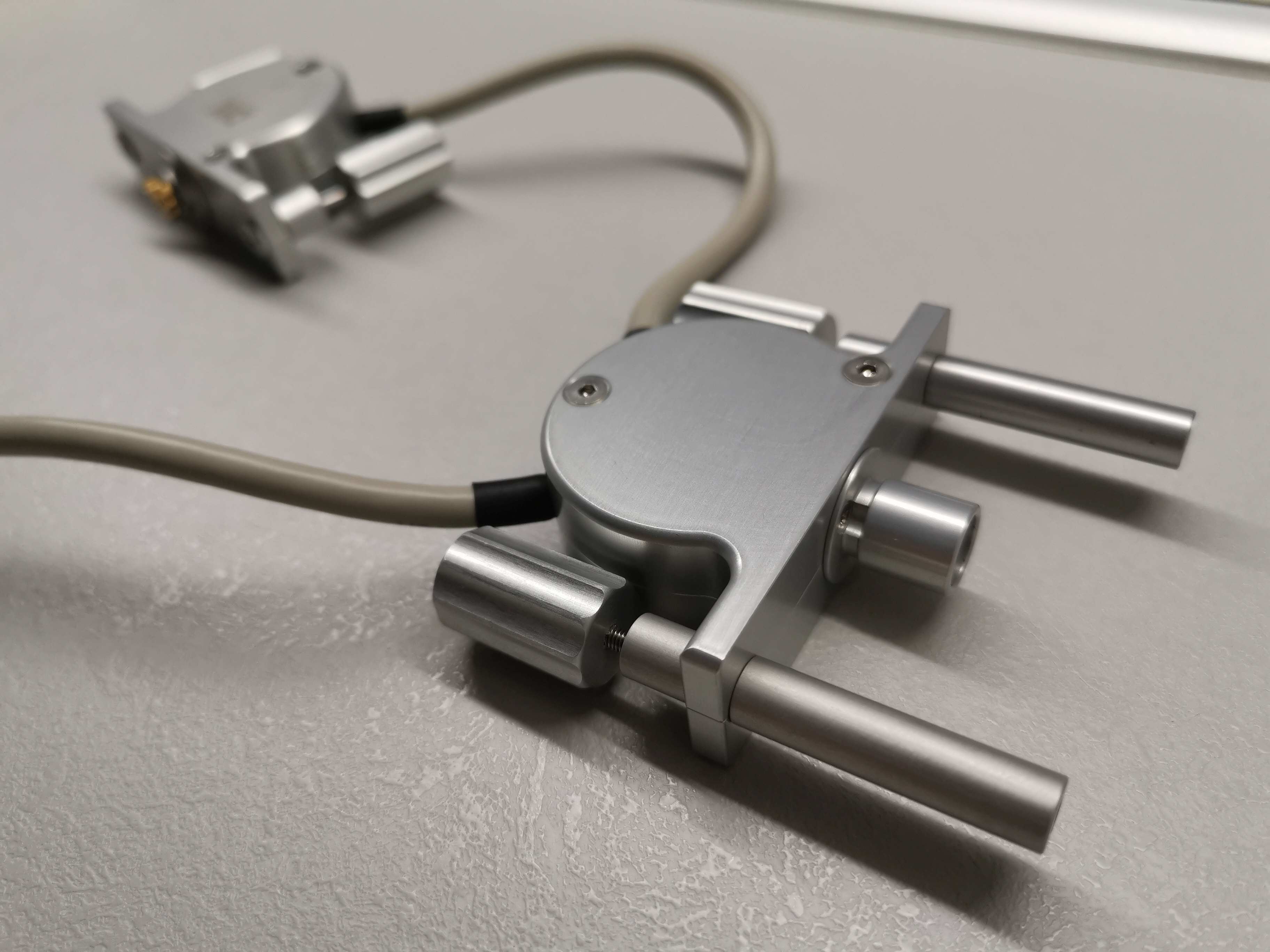
Tracking features
SoLo A includes tracking features that allow for position synchronization with your software. Using an encoder, you can reliably and precisely record the sagital and transversal by employing an encoder, you can accuratly and consistently capture the linear and rotational position of the ultrasound probe.
Our SoLo A system is used successfully in the following processes:
Prostate biopsy
MRI fusion of prostate
HDR/LDR Brachytherapy
Cryotherapy
The SoLo A will be delivered with a modular stepper. Which enables us to guaranty a huge compatibility with the most common endo-rectal ultrasound probes. The compatible ultrasonic systems can be found in the following list. Your system is not listed? Please contact us for a compatibility check with your ultrasound device and ultrasound probe.
Aloka
UST-672-5/7.5 | UST-678-5/7.5
BK Ultrasound
8848 | E14CL4b | 9048 | 8818 | 8808e | 9018
Canon
PVL-715RT
Esaote
TRT 33
Exact Imaging
EV-29L
FujiFilm
EUP-U533 | C41L47RP
GE
ERB | ERB7 | E7C8L-RS
Mindray
6LB7 | 6LB7S
Siemens
Endo-PII
SIUI
ECBP
Sonoscape
Bi-plane ER Probe BCL10-5
Terason
8BP4
SolidLock HS

The medical support arm SolidLock holds its attachements into position. Six independant joints allow a 360° movement.
Stepper A-TP

Together with our A-TP stepper, you will receive a suitable transducer (holder for probe) and an inlay for your ultrasonic probe.
Template holder
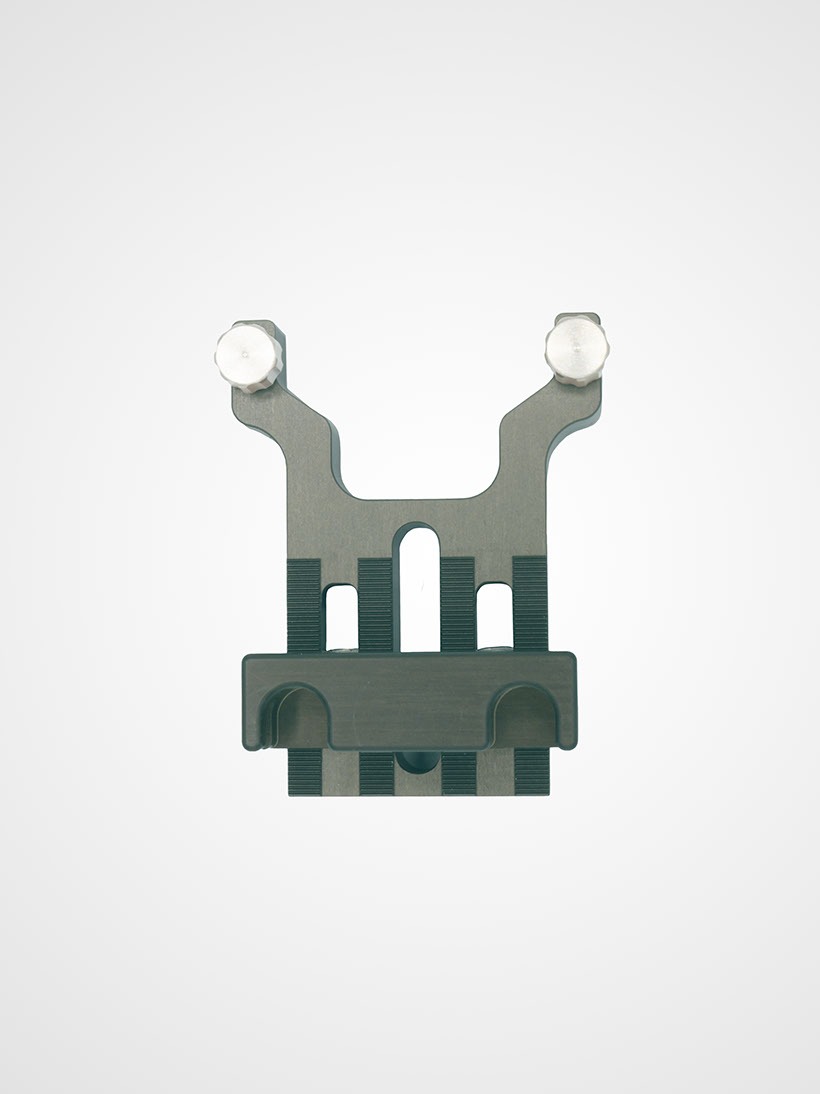
Our template holder allows you to attach guide grids for the secure placement of needles, seed strands and the like.
Interested in our transperineal access system? Contact us now for information!
Contents that may also be engaging:
Don’t need a template or tracking functions? Take a look at our basic positioning system SoLo B!
Access system with stepper for endorectal ultrasound procedures
The system SoLo A is MTT’s solution for a precise positioning of endo-rectal ultrasound probes in TRUS examinations and procedures.
SoLo A will be customized for your ultrasound system
Our steppers are build modular, which allows us to adapt it to your individual specifications. Based on the endo-rectal ultrasound probe you are using, you get a suitable inlay for the transducer (probe holder) and/or a centering element to keep the probe into position. Due to this we can fit our SoLo A to your existing system.
Easy to clean
The transperineal access system SoLo A is easy to clean, because all components of the stepper are fully autoclavable and the included articulated arm SolidLock can be cleaned using wipe disinfection.
Please follow the instructions in the instructions for use on the subject of cleaning and disinfection.
Mobile and takes up little space
SoLo A can be quickly and easily attached to OR bed rails or an optional floor stand. The stand also allows you to position the articulated arm elsewhere. When our trans-perineal access system is not in use, it takes up very little space as the articulated arm can be folded up and stored to save space.
Certified medical articulated arm
The rotatable and adjustable SolidLock arm is specially designed for use by medical professionals in doctors’ practices and operating theaters. There, the SolidLock is attached to a surgical rail or similar holder and positions medical devices relative to the patient. The SolidLock can be used for all body regions and patient groups, except for neurosurgery or direct applications on the central nervous system.
In accordance with EU MDR 2017/745, all SolidLock variants are approved as Class I non-invasive medical devices.
