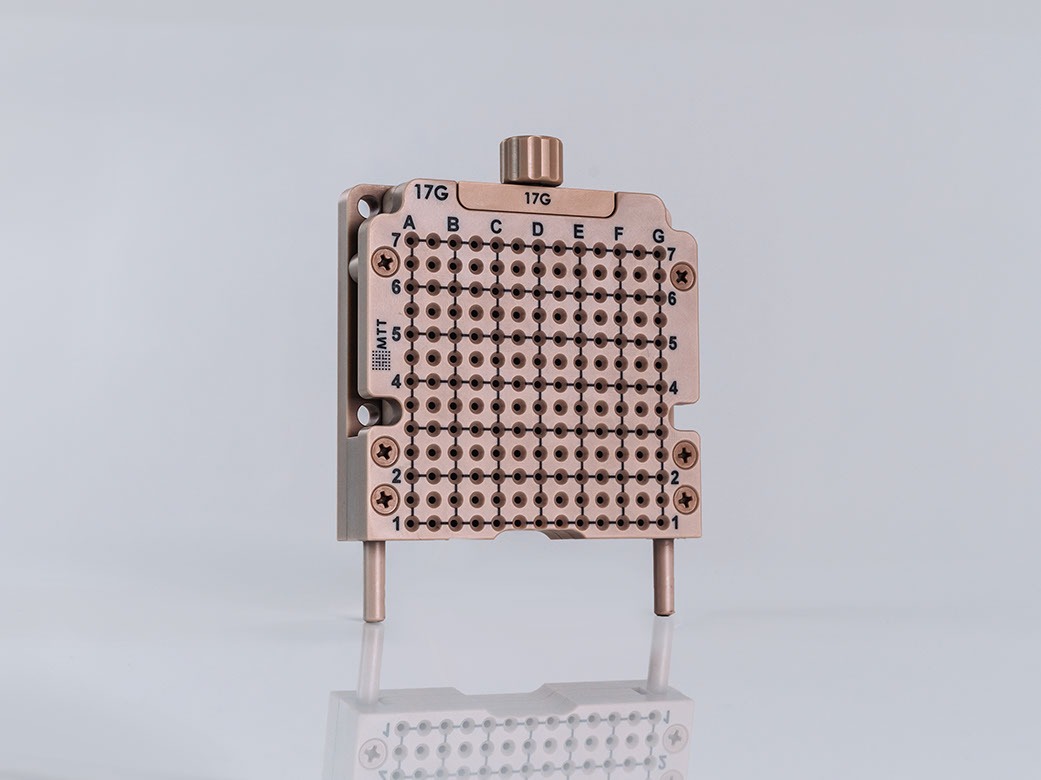
Size Variants
Field of Use
Hole Spacing
5 mm vertical, 5 mm horizontal
Scala
A-G; 1-7
Measurement
85 x 85 x 20 mm, 200 g
Materials
Stainlees steel 1.4301 / 1.4305
Our PeriGrid HDR has an additional fixation plate to hold the needle in place during longer applications and two lateral eyelets for suturing to the patient’s perineum. It is also MR safe.
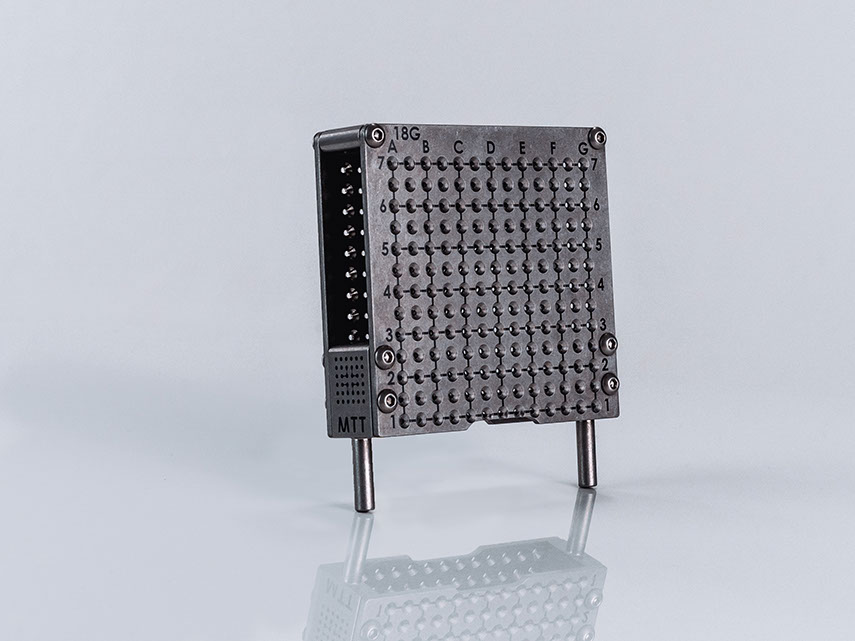
Size Variants
Field of Use
Hole Spacing
5 mm vertikal, 5 mm horizontal
only 17G Spezial 0.33:
3,3 mm vertical, 3,3 mm horizontal, 19×19 holes
Scala
A-G; 1-7
Measurement
85 x 85 x 20 mm; 150 g
Materials
PEEK
The template features two additional rows of openings for lateral and dorsal needle placements. The grid is for single use only and is sterile packed.
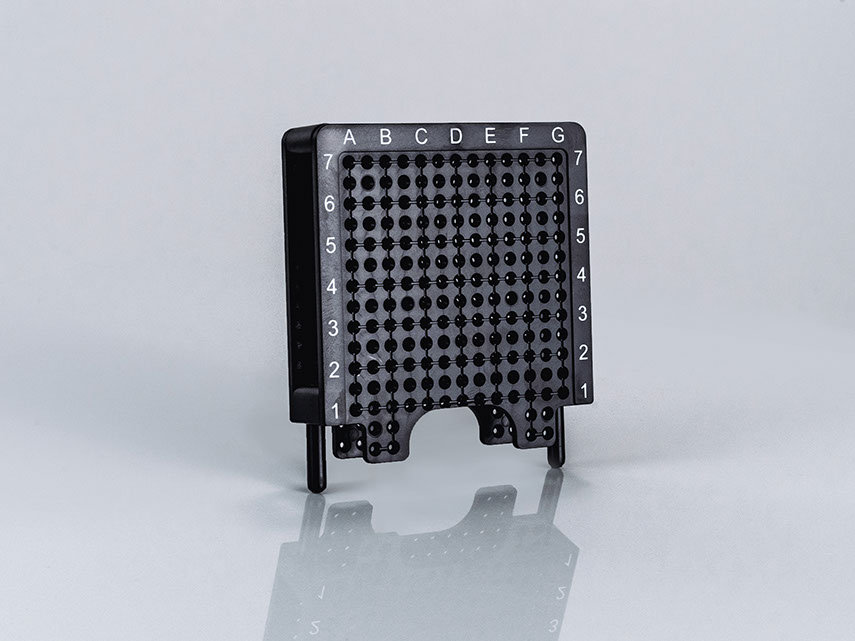
Size Variants
Field of Use
Hole Spacing
5 mm vertical, 5 mm horizontal
Scala
A-G; 1-7
Measurement
90 x 78 x 18 mm, 30 g
Materials
Polycarbonate
Considering PeriGrid for your applications? Get in touch with us!
This content may appeal to you as well:
Medical purpose
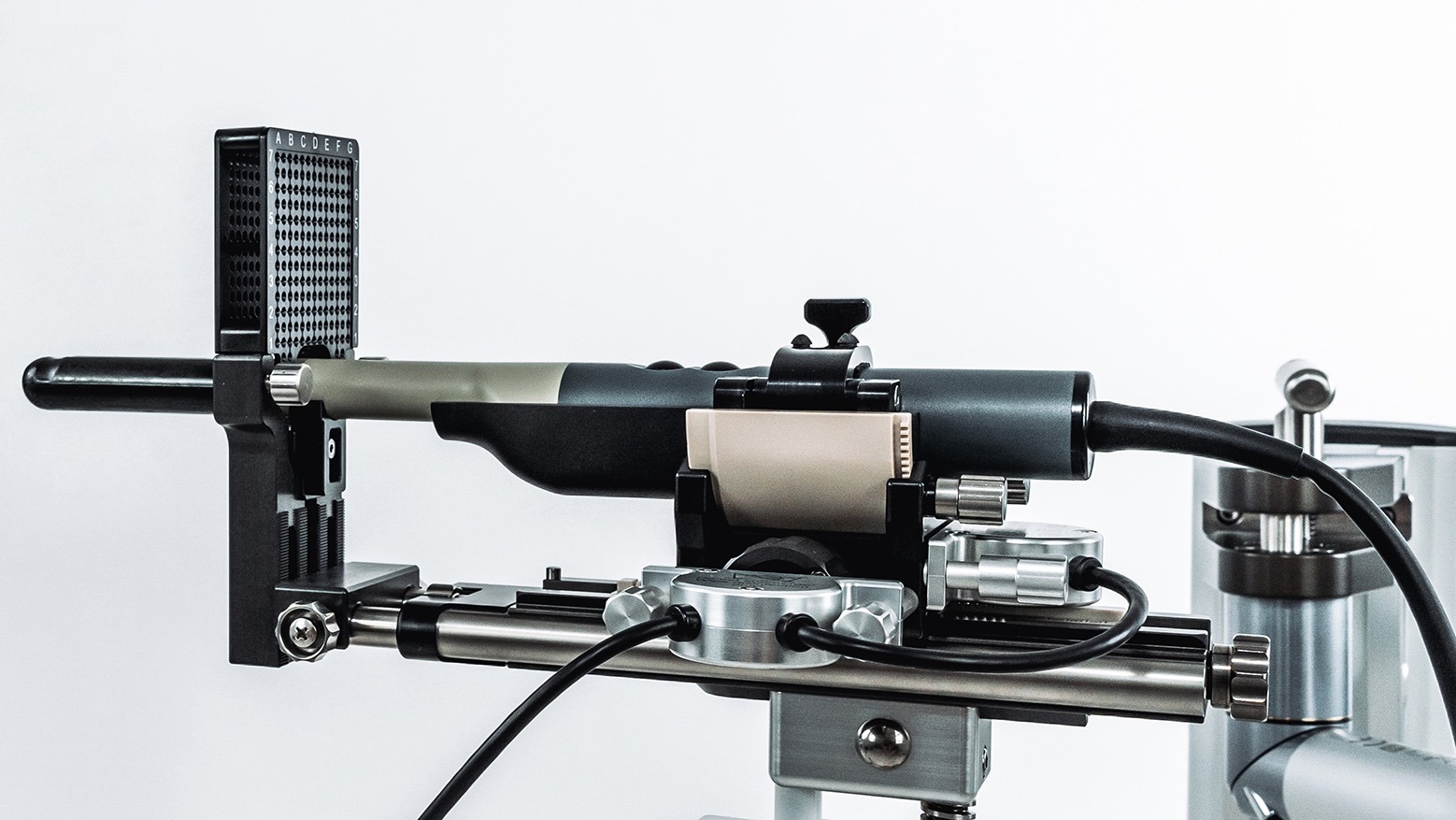
Our PeriGrid is used to assist in manual needle positioning during ultrasound-guided transperineal prostate biopsies or focal therapies (such as cryotherapy), as well as LDR and HDR brachytherapy of the prostate. The guide grid is placed on the template holder of the SoLo A positioning system and secured. The selected needle is then inserted through the desired guide hole on the front and back of the grid.
Do not use alone – additional equipment required for use
PeriGrids can only be used with a template holder! This must correspond with the lower measurements of the grids and be flexible. This has been validated for our internal holder for the Stepper A-TP. Employ biplanar endorectal probes while implementing PeriGrid. Please be aware of the ultrasound probes that are suitable for the SoLo A.
Certified needle guide for medical use
The appropriate use of our PeriGrid is limited to the operating room and urological offices. In accordance with EU MDR 2017/745, PeriGrid Disposable and Reusable are approved as Class IIa non-invasive medical devices for temporary use. And our PeriGrid HDR is approved as a Class IIa non-invasive medical device for short-term use.